Clinical examples
In clinical trials, XHANCE significantly improved nasal congestion at Week 4 and significantly reduced bilateral polyp grade at Week 16 (coprimary endpoints)1:
- Average reduction in congestion using a 0-3 point scale: 0.62 XHANCE 372 mcg BID vs 0.24 EDS-placebo (P<0.001)
- Average reduction of polyp grade in a clinical trial using a 0-3 point scale: 1.41 XHANCE 372 mcg BID vs 0.61 EDS-placebo (P<0.001)
Endoscopic video examples of the impact of XHANCE
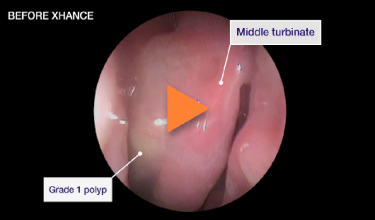
Patient 1
Reduction from grade 2 to grade 1
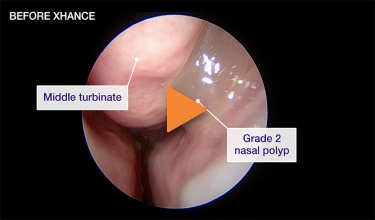
Patient 2
Reduction from grade 2 to grade 0
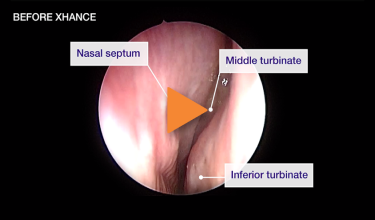
Patient 3
Improvement in SNOT-22 score
These results may not be representative of all patients using XHANCE.
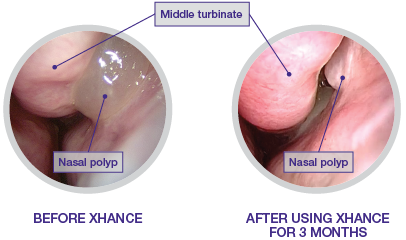
A side-by-side example2
- Patient experienced polyp recurrence following endoscopic sinus surgery
- Grade 2 nasal polyp located in the left nasal cavity confirmed by rigid endoscopy
- Patient was not taking any medication for the treatment of nasal polyps but was being treated for asthma
The clinical trials for XHANCE evaluated over 1500 patients.2
See what makes XHANCE different.