Post hoc subgroup analysis Post hoc analysis of NAVIGATE I and II
Reference:
POLYP REDUCTION IN PATIENTS PREVIOUSLY USING STANDARD NASAL STEROIDS1
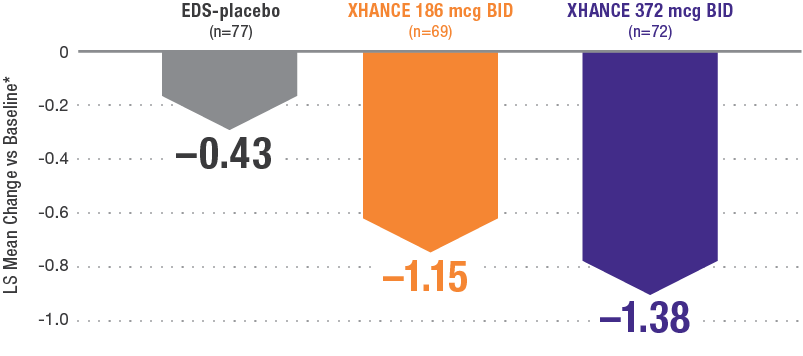
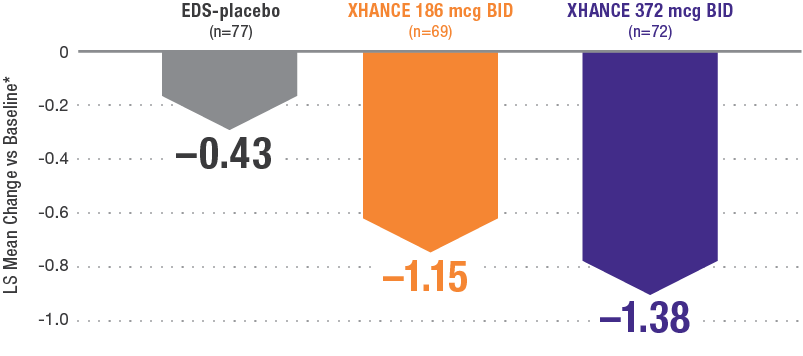
Reductions in nasal polyp grade at Week 16 in the subgroup of patients reporting standard nasal steroid use prior to study entry1
In a subgroup of patients reporting use of a standard nasal steroid within 30 days of the screening visit1:
- Patients reported a mean treatment duration of 3 years with a standard nasal steroid
- The improvements in this subgroup were similar in magnitude to improvements in overall study population
Post hoc analysis of NAVIGATE I and II
Pooled subgroup data (n=218)


*Polyp grade was determined by the clinician using nasal endoscopy. Polyps on each side of the nose were graded on a categorical scale (0=No polyps; 1=Mild: polyps not reached below the inferior border of the middle turbinate; 2=Moderate: polyps reaching below the inferior border of the middle concha, but not the inferior border of the inferior turbinate; 3=Severe: large polyps reaching below the lower inferior border of the inferior turbinate1
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS: - Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
Please see additional Important Safety Information and full Prescribing Information, including Instructions for Use.
- Senior BA, Schlosser RJ, Bosso, J, Soler ZM. Efficacy of the exhalation delivery system with fluticasone in patients who remain symptomatic on standard nasal steroid sprays. Int Forum Allergy Rhinol. 2020.