Post hoc subgroup analysis
Improvement in SNOT-22 in patients previously
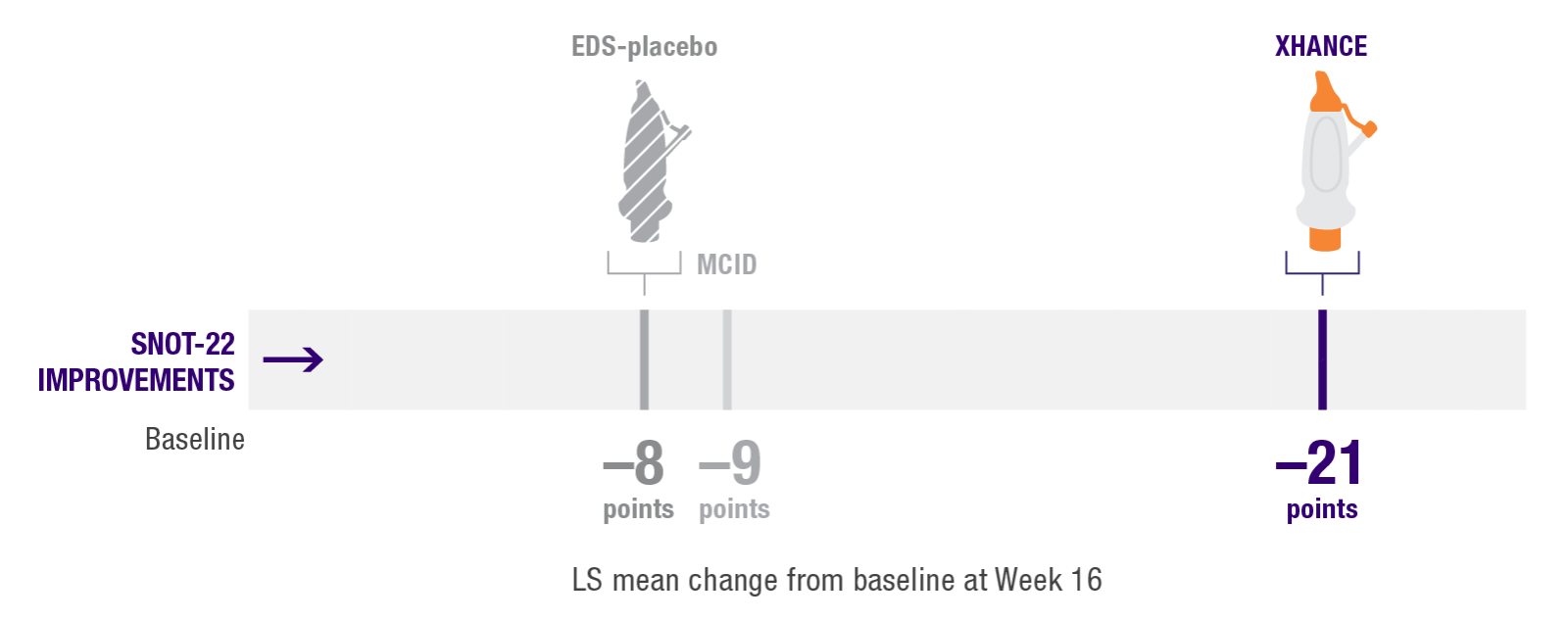
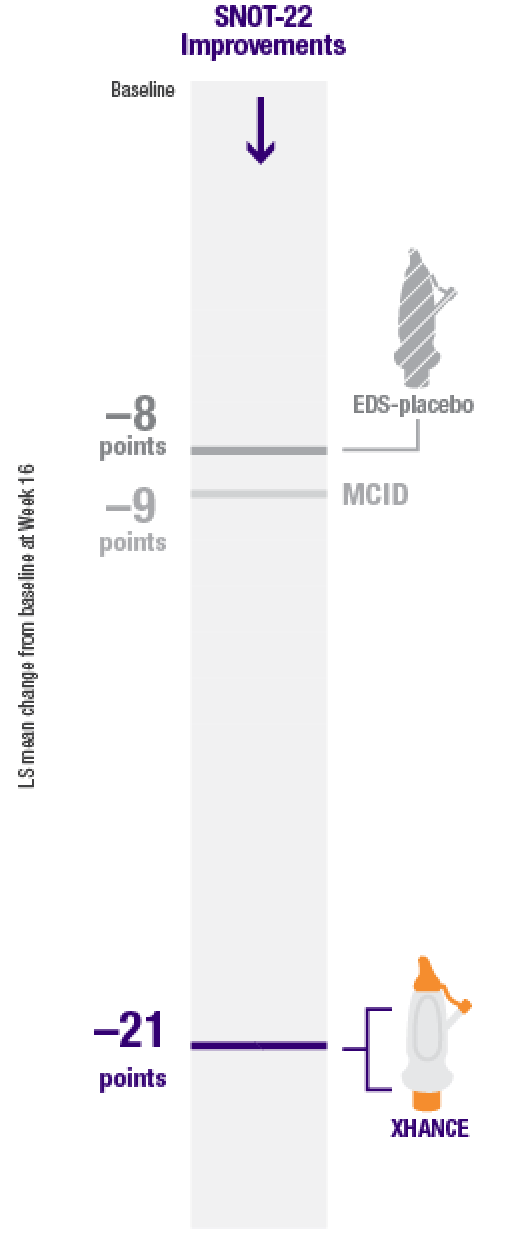
Mean SNOT-22 scores improved by 21 points at Week 16 in patients taking XHANCE (both the 186 mcg BID dose and the 372 mcg BID dose)1
Minimal clinically important difference (MCID) of –9 is considered a clinically relevant improvement2,3
In the clinical trials for XHANCE, the mean baseline SNOT-22 score for all arms was ~501,4,5
In a subgroup of patients reporting use of a standard nasal steroid within 30 days of the screening visit1:
The results shown below are descriptive and should be interpreted with caution.
Post hoc analysis of NAVIGATE I and II
References:
Improvement in SNOT-22 in patients previously
using standard nasal steroids1
Improvements in SNOT-22 scores in the subgroup of patients reporting use of standard nasal steroids prior to study entry1
– Patients reported a mean treatment duration of 3 years with a standard nasal steroid
Post hoc analysis of NAVIGATE I and II
Pooled subgroup data (n=218)


SNOT-22 is a questionnaire for assessing symptoms, quality of life, and functioning. Patients report answers to 22 questions using a scale from 0 (“no problem”) to 5 (“problem as bad as can be”). Responses are summed, and total score ranges from 0 to 110, with a mean score of 9.3 for healthy patients.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS:
- Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
Please see additional Important Safety Information and full Prescribing Information, including Instructions for Use.
- Senior BA, Schlosser RJ, Bosso J, Soler ZM. Efficacy of the exhalation delivery system with fluticasone in patients who remain symptomatic on standard nasal steroid sprays. Int Forum Allergy Rhinol. 2021;11(5):837-845.
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447-454.
- Le PT, Soler ZM, Jones R, Mattos JL, Nguyen SA, Schlosser RJ. Systematic review and meta-analysis of SNOT-22 outcomes after surgery for chronic rhinosinusitis with nasal polyposis. Otolaryngol Head Neck Surg. 2018;159(3):414-423.
- Leopold DA, Elkayam D, Messina JC, Kosik-Gonzalez C, Djupesland PG, Mahmoud RA. NAVIGATE II: randomized double-blind trial of the exhalation delivery system with fluticasone(EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the exhalation delivery system with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.