Post hoc subgroup analysis Post hoc analysis of NAVIGATE I and II

References:
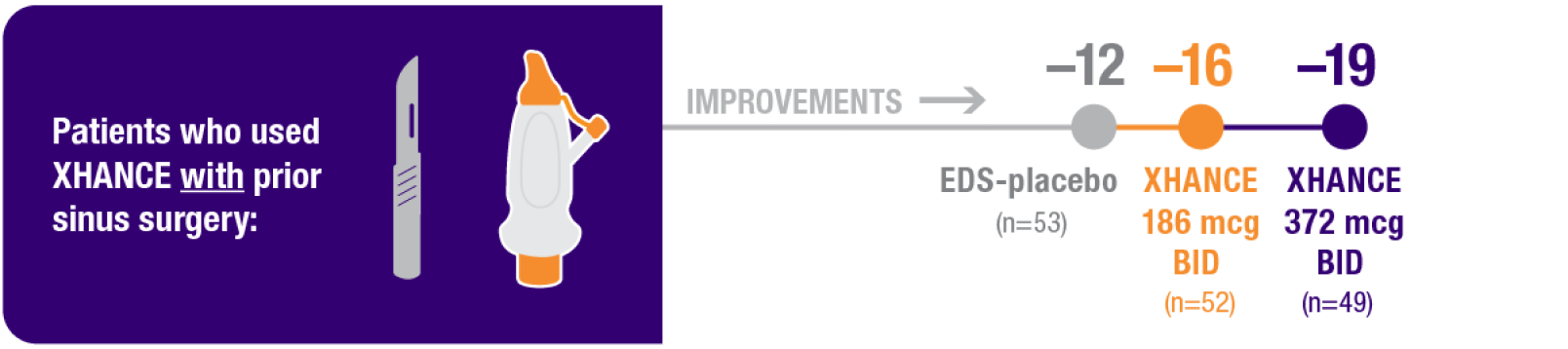
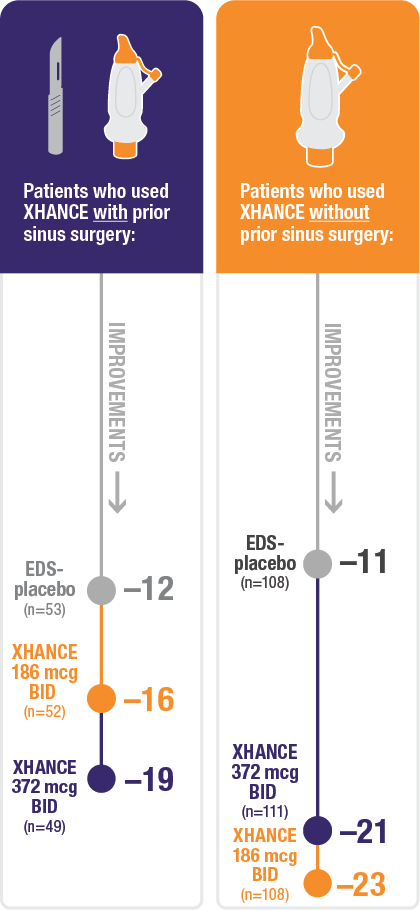
Improvement in SNOT-22 in patients with or without prior sinus surgery
Reduction in SNOT-22 score at Week 16 in the subgroups of patients with or without prior sinus surgery1
- A post hoc analysis of a pooled population from NAVIGATE I and NAVIGATE II analyzed improvement in SNOT-22 score in the subgroup of patients who had sinus surgery and those without sinus surgery prior to study entry1
- Patients were excluded from the studies if they had a history of sinus or nasal surgery within 6 months before screening2,3
Post hoc analysis of NAVIGATE I and II
Pooled subgroup data (n=481)



Baseline grade: EDS-placebo, 3.7; XHANCE 186 mcg BID, 3.8; XHANCE 372 mcg BID, 3.7.1-3
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS: - Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
Please see additional Important Safety Information and full Prescribing Information, including Instructions for Use.
- Data on file. OptiNose US, Inc.
- Leopold DA, Elkayam D, Messina JC, et al. NAVIGATE II: randomized double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126-134.
- Sindwani R, Han JK, Soteres DF, et al. NAVIGATE I: randomized, placebo-controlled, double-blind trial of the Exhalation Delivery System with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33(1):69-82.